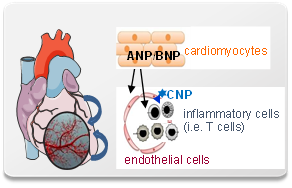
ANP-mediated cardiomyocyte-endothelial cell cross-talk: role in ischemia/reperfusion-induced activation of immune cells (B2)
Natriuretic peptides in cardiac ischemia-reperfusion
One of the most rapid and robust responses of cardiomyocytes to ischemia-reperfusion is the increased expression of the natriuretic peptides (NPs) ANP and BNP. This is accompanied by endothelial CNP release. Coronary endothelial cells co-express three NP receptors: the cyclic GMP-forming guanylyl cyclase (GC) receptors A (GC-A, shared by ANP and BNP) and B (GC-B, selective for CNP); and the NP receptor C (NPR-C), binding the three hormones with similar affinities. The role of these receptors in the regulation of coronary endothelial barrier functions and immune cell extravasation is unknown.
In the systemic circulation, ANP, via endothelial GC-A/cGMP signalling, mildly increases albumin permeability. The resultant shift of intravascular fluid to the interstitial space prevents hypervolemia.
In contrast, CNP, via GC-B, generally strengthens the anti-inflammatory properties of the endothelium. The role of NPR-C is controversial. Some studies indicated that it mediates NP´s internalization and “clearance”; others that it couples to inhibitory G-proteins and mediates harmful endothelial effects of these hormones.
Rationale and aims
This project aims to dissect the effects of NPs on coronary microcirculatory endothelial cells. We hypothesize that physiologically NPs improve their barrier functions. Notably, hypoxia and cytokines (TNF) increase the expression of NPR-C and of the cGMP-stimulated cAMP-degrading phosphodiesterase (PDE) type 2. Thus in cardiac ischemia-reperfusion high local NP levels together with altered endothelial NP signalling might decrease endothelial cAMP levels and thereby contribute to microvascular dysfunction with subsequent activation of immune cells.
Approach
We will study cultured myocardial endothelial cells and novel genetic mouse models with endothelial deletion of single components of NP signalling pathways. This will decipher the differential effects of ANP/BNP versus CNP on endothelial barrier properties in cardiac physiology and disease. The results may help to identify targets for therapies directed to prevent or decelerate coronary microvascular injury and inflammation after reperfused AMI.
Significance and outlook
We expect that this approach will enable us to decipher the differential effects of these locally produced hormones on the coronary microvascular endothelium and endothelial-immune cell interactions. The result may help to identify targets for therapies directed to attenuate coronary microvascular injury and myocardial inflammation after reperfused AMI.
Anschrift
Medizinische Klinik und Poliklinik I, Universitätsklinikum Würzburg, Zentrum für Innere Medizin (ZIM), Oberdürrbacher Straße 6, Haus A3, 97080 Würzburg, Deutschland
Deutsches Zentrum für Herzinsuffizienz Würzburg | Comprehensive Heart Failure Center | Am Schwarzenberg 15 | Haus A15 | 97078 Würzburg