Selective PET radiotracers as specific biomarkers (C1)
Background
Neutrophils are part of the innate immune system and migrate rapidly from blood into the inflamed tissue. Therefore, neutrophils are considered as key players in acute inflammation. However, recent studies suggest beneficial effects of neutrophils for cardiac healing and outcome. Therefore, neutrophils’ roles in acute post MI inflammation, such as beneficial vs. damaging effects and their time courses, are still unknown. To gain a deeper understanding of neutrophils with regards to therapeutic treatment, we want to monitor their migration via positron emission tomography (PET), a non-invasive imaging tool using a radiolabeled marker (radiotracer). For this purpose, a radiotracer that specifically binds on neutrophils would be ideal. In general, such a radiotracer persists of a high affinity ligand that is attached to a radionuclide. As a radionuclide we aim to use fluorine-18 since it possesses a comparingly long half life time (110 min) and can be produced directly in the clinical facility.
Aim
We aim to develop a non-invasive diagnostic tool that allows us to monitor the localization and the intensity of neutrophil infiltration at different interfaces and stages of the inflammation process, e.g., after MI. Therefore, “cold” and “hot” (fluorine-18) derivatives of known ligands will be synthesized and evaluated in vitro and in vivo.
Approach
For the development of a such a tracer, a “cold”/unlabeled ligand that specifically binds on neutrophils has to be identified first. Therefore, we use literature known neutrophil-binding ligands as our lead structure where we insert fluorine atoms in different positions of the molecule. The obtained set of synthesized compounds is then tested in a radioligand binding assay on their target-binding ability as well as selectivity in a NanoBRET assay. The compound with the best affinity and selectivity values will be selected and the respective precursor for radiolabelling will be synthesized. This precursor is then labelled to achieve the “hot”/labelled ligand which will then be tested on their binding behavior on animal and human neutrophils respectively. Finally, the tracer will be injected in an animal model to determine its biodistribution and clearance. Observed deficits in the radiotracer’s in vivo behavior will then be adjusted back on its molecular structure level and the respective synthesis of ligands and their biological evaluation restarts in an iterative approach if necessary.
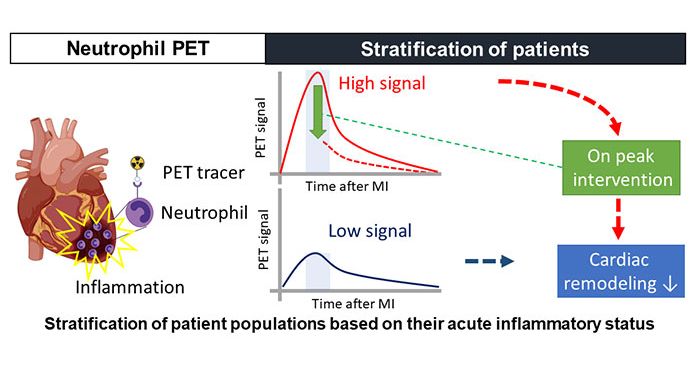
Significance and Outlook
A suitable radiotracer will give us a deeper understanding of the neutrophils role during the acute heart inflammation after a MI. The knowledge gained can then be transferred into the optimization of treatment strategies.
Anschrift
Medizinische Klinik und Poliklinik I, Universitätsklinikum Würzburg, Zentrum für Innere Medizin (ZIM), Oberdürrbacher Straße 6, Haus A3, 97080 Würzburg, Deutschland
Deutsches Zentrum für Herzinsuffizienz Würzburg | Comprehensive Heart Failure Center | Am Schwarzenberg 15 | Haus A15 | 97078 Würzburg



