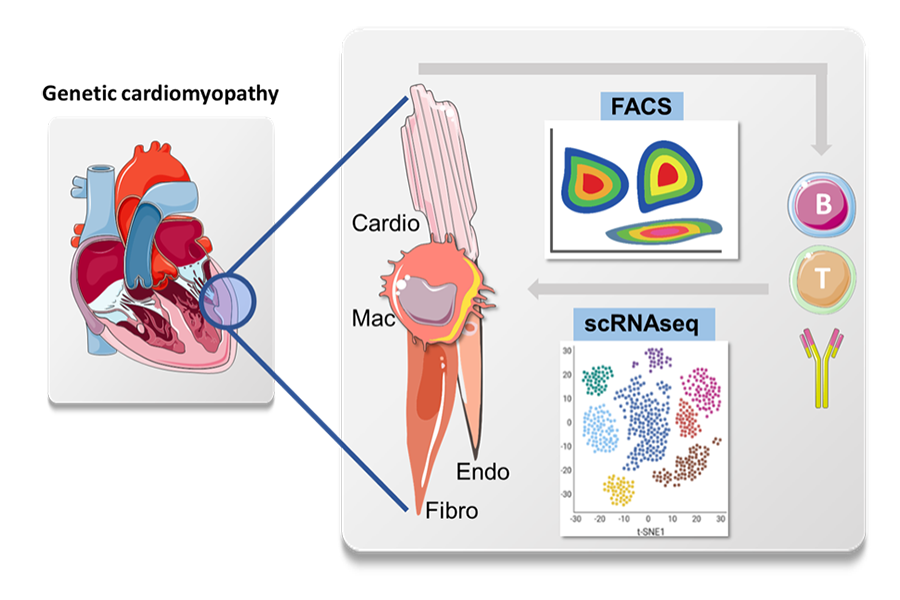
Cardiac pathology in Duchenne disease
Duchenne muscular dystrophy is an inherited disease which primarily manifest as progressive skelettal muscle weakness. The disease affects about one in 5,000 males at birth and is the most common type of muscular dystrophy. Loss of function mutations in the dystrophin gene cause a progressive loss of myocytes in the heart and skelettal muscle. The cardic myopathy becomes mostly apparent later as heart failure. There are numerous disease relevant rezessive mutations in the X-chromosomal Dystrophin gene described making gene therapy challenging. At present no curative treatment option is available.
Rationale and aims
Steroid therapy is known to improve peripheral muscle strength and to slow disease progression. Our own data indicate that lymphocytes are causally involved in the manifestation of the cardiac phenotype in an animal model. Building on these observations we aim to study how ad when the inflammation in the myocardium is initiated and how T-cells, B-cells and antibodies differentially contribute to the evolution of the cardiomyopathy.
Approach
We will characterize leukocytes within the myocardium using single-cell sequencing approaches (scRNAseq) and flow cytometry (FACS) at various stages of the disease to understand how lymphocytes interact withmyeloid cells, endothelial cells and fibroblasts. Moreover, using transcriptomic approaches and in vitro studies, we want to understand how cardiomyocyte dysfunction initiates local inflammation and leukocyte attraction.
Significance and outlook
We hypothesize that some mechanism we encounter in this cardiomyopathy model apply to other inherited cardiomyopathies. Lymphocyte targeting therapies might be able to slow down the disease course and prevent from heart failure morbidity in affected individuals.
Anschrift
Medizinische Klinik und Poliklinik I, Universitätsklinikum Würzburg, Zentrum für Innere Medizin (ZIM), Oberdürrbacher Straße 6, Haus A3, 97080 Würzburg, Deutschland
Deutsches Zentrum für Herzinsuffizienz Würzburg | Comprehensive Heart Failure Center | Am Schwarzenberg 15 | Haus A15 | 97078 Würzburg