Core Facilitiy for Cellular Electrophysiology
The core technique is fluorescence microscopy combined with either external field stimulation, the patch-clamp technique or cellular force measurements. With these techniques, we can analyze all relevant processes of EC coupling (ion currents, action potentials, calcium and sodium fluxes, cell shortening, diastolic tension and systolic force generation) in parallel with key bioenergetics parameters, such as mitochondrial calcium uptake, the redox state (of NADH, NADPH and FAD), mitochondrial membrane potential and formation of reactive oxygen species (ROS).
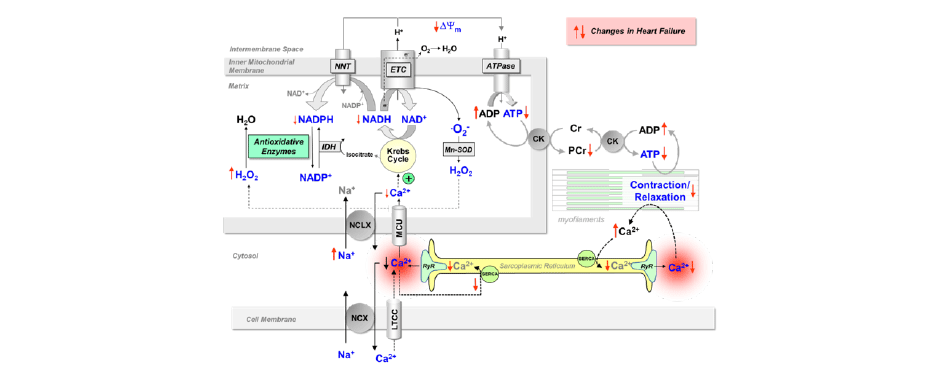
Scheme: Processes of EC coupling and mitochondrial energetics in cardiac myocytes. All processes highlighted in blue can be monitored with our techniques.
IonOptix set-up

IonOptix set-up with 3-fold photomultiplier tube assembly for combining fluorescence measurements with sarcomere löength determination for detection of parameters highlighted in the Scheme. This set-up serves high-throughput analysis of unloaded intact cardiac myocytes during EC coupling. (Kohlhaas & Maack, 2010; Chen et al., 2012; Nickel et al., 2015; Kohlhaas et al., 2017).
Patch-clamp set-up

Patch-clamp set-up with integrated 3-fold photomultiplier tube assembly to combine ion current (or action potential) with fluorescence measurements. With this set-up, we can employ a technique for quite specific determination of cytosolic and mitochondrial calcium handling in the same cells, respectively (Maack et al., 2006; Kohlhaas et al., 2010; Kohlhaas & Maack, 2010).
IonOptics force measurement set-up

Myocytes are attached to glass rods (see small inset) and pre-stretched to a physiological length, at which they can exert isometric, isotonic or auxotonic contractions. Together with sarcomere length and force generation, fluorescence parameters of EC coupling and mitochondrial parameters (see Scheme) can be analyzed.
Contact Person
Prof. Dr. med.
Christoph Maack (Translationale Forschung)
Leiter des Departments Translationale Forschung
+49 931 201-46502